Measuring principle
When be normally breathing, the gas passed through the flow sensor. On both sides of the screen of the sensor will produce different pressure. The pressure is detected by the sensor, which is converted to electrical signals. After the signal amplification, AD converter sampling, the pressure is converted to digital signal into the computer system.
Through digital signal processing and analyzing technology, computer system reproduces the breathing waves that are displayed on the LCD together with analyzed results. In the end the built-in printer prints out the analysis results.Single use filter
Product Features
Maximum Ventilator Volume (MVV).
110mm thermal printer built-in can print out a clear clinical report predicted value measured value
Product Specifications
Related Accessories
Related Products

iBreathe®
The iBreathe spirometer is a portable device for checking lung function. It uses the principle of differential pressure measurement to measure over 100 parameters related to FVC, VC, MVV
Promoted Products
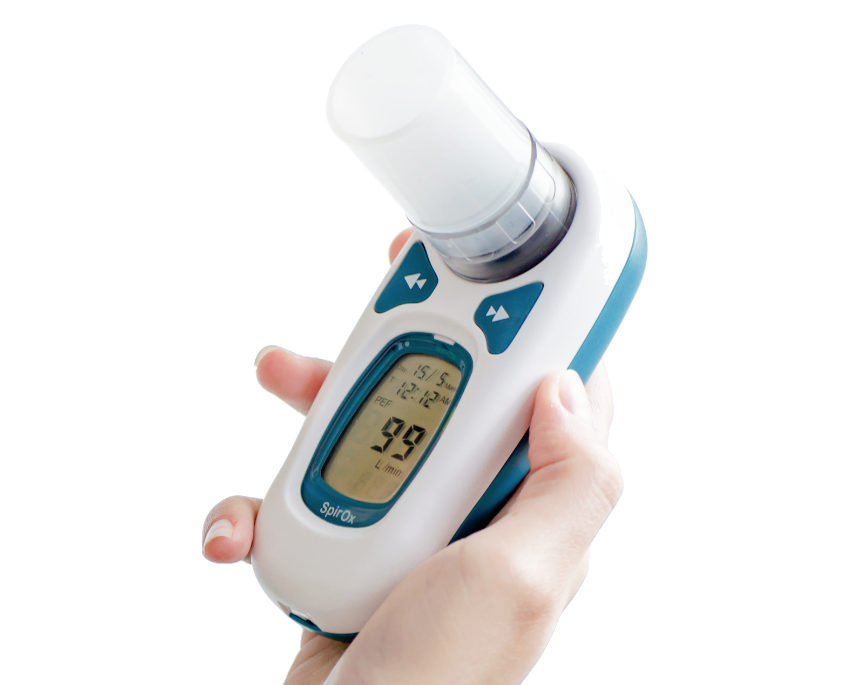
Spirometer Type: P
Palm size Spirometer with LCD screen PEF and FEV1 test Meet ATS American Thoracic Society ,1994 update standardization. Suitable for hospital or home. Maximum of records is 600 measurements
Meditech Equipment Co.,Ltd is part of Meditech Group. Product(s) described may not be licensed or available for sale in all countries. Sonotech, Sonovet, iSonic, FOs2pro, Dolphi, Defi, HeartRec,miniScan,Cardios,SpirOx,iBreath, Meditech and all corresponding design marks are trademarks of Meditech. The symbol indicates the trademark is registered. Patent and Trademark Office and certain other countries. All other names and marks mentioned are the trade names, trademarks or service marks of their respective owners. Please see the Instructions for Use for a complete listing of the indications, contraindications, warnings and precautions.
Legal notice Terms and conditions Cookie policy Privacy Policy Professional organisations Careers